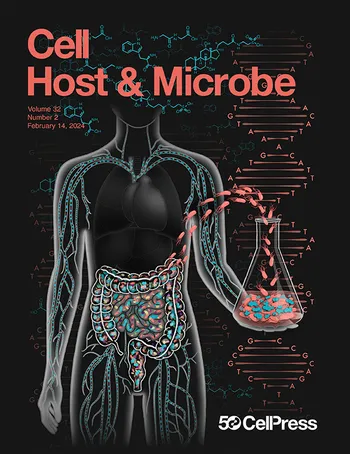
The Schirmer lab uses multi-omics data from large human cohort studies to predict host-microbial interactions associated with disease. The underlying disease mechanisms are then further studied using the respective microbial isolates in combination with gut organoids.
The research of the Schirmer lab is focused on understanding the role of the human microbiome in inflammatory and immune-related diseases. The microbiome refers to the large collection of bacteria, viruses, archaea and fungi that live in and on our body. Most of these organisms live in our gut and provide important immunological and metabolic benefits. In many diseases, such as chronic inflammatory bowel diseases and immune-related diseases, an imbalance of these microbial communities has been observed. The underlying reasons and consequences of this imbalance are largely unknown though.
We investigate how these microbes communicate and interact with their human host and identify aberrant host-microbial interactions that play a role in disease. For this, we start from patient samples and generate state-of-the-art multi-omics data, including metagenomics, metatranscriptomics and metabolomics data. We subsequently analyse these datasets to infer microbiome profiles. Using integrated analysis with clinical and patient metadata, we can computationally identify disease-associated host-microbial interactions. We then further study these hypotheses in the lab and experimentally validate the immunogenicity and inflammatory activity of the identified bacterial strains and metabolites.
We are particularly interested in the gut microbiome and host-microbial interactions that lead to intestinal barrier disruption. For this we use gut organoids as a model to better understand how these disease-associated microbes and microbial products affect gut barrier integrity. Ultimately, we want to leverage this knowledge to improve our ability to diagnose, treat and prevent these diseases with new microbiome-based approaches that aim at restoring the host-microbial balance.
Currently our main research projects are focused on:
The role of the oral microbiome in the pathogenesis of gastrointestinal diseases
HEROINE: Hormone-microbiome interactions as a key-player in female health
Next generation drug design: Targeting interkingdom signaling in cancer, inflammatory and infectious diseases
Impact of Desulfovibrio spp. and sulfur metabolism on the pathogenesis of chronic intestinal inflammation and colitis-associated cancer
Our second lab location is at the TUM School of Life Sciences on the Campus in Weihenstephan.
Prof. Dr. Melanie Schirmer (Principal Investigator)
Dr. Sudip Das (Lab Manager)
Dr. Tingting Zheng (Postdoc)
Dr. Mariia Lupatsii (Postdoc)
Shen Jin (PhD student)
Aurelie Cenier (PhD student)
Agnieska Gaska (PhD student)
Svenja Schorlemmer (PhD student)
Emilio Garcia (PhD student)
Jagadrupa Sahoo (PhD student)
Sofie Schönhals (Technician)
Linking microbial genes to plasma and stool metabolites uncovers host-microbial interactions underlying ulcerative colitis disease course. M. Schirmer*#, M. Stražar#, J. Avila-Pacheco, D.F. Rojas-Tapias, E.M. Brwon, E. Temple, A. Deik, K. Bullock, S. Jeanfavre, K. Pierce, S. Jin, R. Invernizzi, M.-M. Pust, Z. Costliow, D.R. Mack, A.M. Griffiths, T. Walters, B.M. Boyle, S. Kugathasan, H. Vlamakis, J. Hyams, L. Denson, C.B. Clish, R.J. Xavier*. Cell Host & Microbe (2024) *co-corresponding authors, #contributed equally
Inflammation-associated nitrate facilitates ectopic colonization of oral bacterium Veillonella parvula in the intestine. D.F. Rojas-Tapias, E.M. Brown, E.R. Temple, M.A. Onyekaba, A.M.T. Mohamed, K. Duncan, M. Schirmer, R.L. Walker, T. Mayassi, K.A. Pierce, J. Avila-Pacheco, C.B. Clish, H. Vlamakis, R.J. Xavier. Nature Microbiology (2022)
Deciphering mechanisms and implications of bacterial translocation in human health and disease. S. Jin, D. Wetzel, M. Schirmer. Current Opinion in Microbiology (2022)
Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. J. Lloyd-Price, C. Arze, A.N. Ananthakrischnan, M. Schirmer, J. Avila-Pacheco, T.W. Poon, E. Andrews, N.J. Ajami, K.S. Bonham, C.J. Brislawn, D. Casero, H. Courtney, A. Gonzalez, T.G. Graeber, A.B. Hall, K. Lake, C.J. Landers, H. Mallick, D.R. Plichta, M. Prasad, G. Rahnavard, J. Sauk, D. Shungin, Y. Vásquez-Baeza, R.A. White III, IBDMDB Investigators, J. Braun, L.A. Denson, J.K. Jansson, R. Knight, S. Kugathasan, D.P.B. McGovern, J.F. Petrosino, R.S. Stappenbeck, H.S. Winter, C.B. Clish, E.A. Franzosa, H. Vlamakis, R.J. Xavier, C. Huttenhower. Nature (2019)
Species-level functional profiling of metagenomes and metatranscriptomes. E.A. Franzosa, L.J. McIver, G. Rahnavard, LR. Thompson, M. Schirmer, G. Weingart, K.S. Lipson, R. Knight, J.G. Caporaso, N. Segata, C. Huttenhower. Nature Methods (2018)
Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. M. Schirmer, L. Denson, H. Vlamakis, E.A. Franzosa, S. Thomas, N.M. Gotman, P. Rufo, S.S. Baker, C. Sauer, J. Markowitz, M. Pfefferkorn, M. Oliva-Hemker, J. Rosh, A. Otley, B. Boyle, D. Mack, R. Baldassano, D. Keljo, N. LeLeiko, M. Heyman, A. Griffiths, A.S. Patel, J. Noe, S. Kugathasan, T. Walters, C. Huttenhower, J. Hyrams, R.J. Xavier. Cell Host & Microbe (2018)
Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. M. Schirmer, E.A. Franzosa, J. Lloyd-Price, L.J. McIver, R. Schwager, T.W. Poon, A.N. Ananthakrishnan, E. Andrews, G. Barron, K. Lake, M. Prasad, J. Sauk, B. Stevens, R.G. Wilson, J. Braun, L.A. Denson, S. Kugathasan, D.P.B. McGovern, H. Vlamakis, R.J. Xavier*, C. Huttenhower*. Nature Microbiology (2018) *co-corresponding authors
Illumina error profiles: resolving fine-scale variation in metagenomic sequencing data. M. Schirmer*, R. D'Amore, U.Z. Ijaz, N. Hall, C. Quince*. BMC Bioinformatics (2016) *corresponding author
Linking the human gut microbiome to inflammatory cytokine production capacity. M. Schirmer, S.P. Smeekens, H. Vlamakis, M. Jaeger, M. Oosting, EA. Franzosa, R. ter Horst, T. Jansen, L. Jacobs, M.J. Bonder, A. Kurilshikov, J. Fu, L.A.B. Joosten, A. Zhernakova, C. Huttenhower, C. Wijmenga, M.G. Netea*, R.J. Xavier*. Cell (2016) *co-corresponding authors
Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. A. Zhernakova*, A. Kurilshikov#, M.J. Bonder#, E.F. Tigchelaar#, M. Schirmer, T. Vatanen, Z. Mujagic, A.V. Vila, G. Falony, S. Vieira-Silva, J. Wang, F. Imhann, E. Brandsma, S.A. Jankipersadsing, M. Joossens, M.C. Cenit, P. Deelen, M.A. Swertz, Lifelines Cohort Study, R.K. Weersma, E.J.M. Feskens, M.G. Netea, D. Gevers, D. Jonkers, L. Franke, Y.S. Aulchenko, C. Huttenhower, J. Raes, M.H. Hofker, R.J. Xavier, C. Wijmenga*, J. Fu*. Science (2016) *co-corresponding authors, #contributed equally
The effect of host genetics on the gut microbiome. M.J. Bonder, A. Kurilshikov, E.F. Tigchelaar, Z. Mujagic, F. Imhann, A.V. Vila, P. Deelen, T. Vatanen, M. Schirmer, S.P. Smeekens, D.V. Zhernakova, S.A. Jankipersadsing, M. Jaeger, M. Oosting, M.C. Cenit, A.A.M. Masclee, M.A. Swertz, Y. Li, V. Kumar, L. Joosten, H. Harmsen, R.K. Weersma, L. Franke, M.H. Hofker, R.J. Xavier, D. Jonkers, M.G. Netea, C. Wijmenga*, J. Fu*, A. Zhernakova*. Nature Genetics (2016) *co-corresponding authors
Full publication list: PubMed, Google Scholar
Prof. Dr. Melanie Schirmer
Professorship for Translational Microbiome Data Integration
melanie.schirmer(at)tum.de
Team Assistant:
sekretariat.mdi(at)ls.tum.de
Lab address:
Gregor-Mendel-Str. 2
85354 Freising