Our laboratory investigates fundamental questions in oncology, spanning from basic biology and translational research to precision oncology. We focus on dynamic cell fate decisions in carcinogenesis and their relation to tumor cell dissemination, metastasis, intratumoral heterogeneity (ITH), and therapy resistance to develop novel therapeutic approaches to target these dynamics and induce vulnerabilities for clinical application.
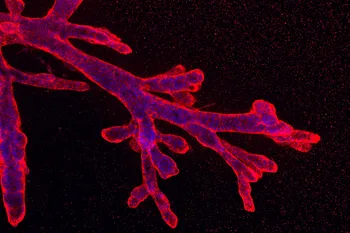
Cell plasticity, the ability of cells to adapt their fate in response to environmental changes, is crucial for tumor adaptation, facilitating tumor initiation, metastasis, heterogeneity, immune escape, and therapy resistance. We have made substantial progress in understanding and leveraging cell plasticity solid cancers, particularly in pancreatic ductal adenocarcinoma (PDAC). By applying modified organoid culture conditions that allow cells to reshape their extracellular matrix (ECM), we identify different PDAC phenotypes recapitulating distinct tumor cell states and intra-tumoral heterogeneity. Using deep convolutional neural networks, we categorize these organoids for large-scale screening to identify novel state-gating and state-targeting therapeutic strategies.
To further study and quantify cancer cell plasticity and states, we use digital holographic microscopy (DHM), which enables a high-throughput characterization of cancer cells label-free and on a single-cell level.
We further corroborate the impact of cancer cell plasticity on tumor development and metastasis using syngeneic and xenogeneic in vivo and in ovo model systems providing insights into the metastatic potential of pancreatic cancer cells and their interaction with the tumor environment.
As translational platform technology, we employ patient-derived organoids (PDOs). PDOs have emerged as models in cancer research, retaining the heterogeneity of primary tumors with predictive value for the clinic. In the Reichert lab, we apply PDOs for validating therapeutic approaches, demonstrating the effectiveness of treatments, and identifying chemotherapy-induced vulnerabilities. Thereby, we can model, identify and exploit treatment-induced plasticity for targeted therapies, providing a mechanistic layer to precision oncology, particularly for highly plastic tumors like pancreatic cancer. In addition, using cell-free DNA (cfDNA) from PDO supernatants allows us early genetic profiling of tumors, facilitating rapid and feasible molecular characterization even from limited biopsy material, which makes PDOs particularly valuable for real-time therapeutic decisions.
The combined use of our advanced model systems and technologies significantly impacts pancreatic cancer research and treatment strategies. By better understanding cell plasticity and leveraging these innovative tools, our ultimate goal is to develop more effective therapeutic approaches for combating highly adaptable and aggressive tumors. These advancements offer new avenues for precision medicine, improving prognosis and treatment outcomes for cancer patients.
Prof. Dr. Maximilian Reichert
Lisa Fricke, M.Sc. (Research associate)
Claudia Guggenmos (Technical assistant)
Dr. Valentina Leone (Postdoc)
Dr. Janine Murr (Postdoc)
Aristeidis Papargyriou, M.Sc. (PhD student)
Dr. Raphela Ranjan (Postdoc)
Aashreya Ravichandra, M.Sc. (PhD student)
Arlett Schäfer (MD student)
Sophia Schirmer (MD student)
Dr. Laura Schmidleitner (Lab manager/Postdoc)
Akul Shastri, M.Sc. (PhD student)
Dr. Martin Troßbach (Postdoc)
Sabrina Wenderoth (Technical assistant)
Heterogeneity-driven phenotypic plasticity and treatment response in branched-organoid models of pancreatic ductal adenocarcinoma. Papargyriou A, Najajreh M, Cook DP, Maurer CH, Bärthel S, Messal HA, Ravichandran SK, Richter T, Knolle M, Metzler T, Shastri AR, Öllinger R, Jasper J, Schmidleitner L, Wang S, Schneeweis C, Ishikawa-Ankerhold H, Engleitner T, Mataite L, Semina M, Trabulssi H, Lange S, Ravichandra A, Schuster M, Mueller S, Peschke K, Schäfer A, Dobiasch S, Combs SE, Schmid RM, Bausch AR, Braren R, Heid I, Scheel CH, Schneider G, Zeigerer A, Luecken MD, Steiger K, Kaissis G, van Rheenen J, Theis FJ, Saur D, Rad R, Reichert M. Nature Biomedical Engineering (2024)
Resistance to mesenchymal reprogramming sustains clonal propagation in metastatic breast cancer. Saini M, Schmidleitner L, Moreno HD, Donato E, Falcone M, Bartsch JM, Klein C, Vogel V, Würth R, Pfarr N, Espinet E, Lehmann M, Königshoff M, Reitberger M, Haas S, Graf E, Schwarzmayr T, Strom TM, Spaich S, Sütterlin M, Schneeweiss A, Weichert W, Schotta G, Reichert M, Aceto N, Sprick MR, Trumpp A, Scheel CH Cell Reports (2023)
Spatiotemporal dynamics of self-organised branching pancreatic cancer-derived organoids. S. Randriamanantsoa*, A. Papargyriou*, C. Maurer, K. Peschke, M. Schuster, K. Steiger, R. Öllinger, D. Saur, C. Scheel, R. Rad, E. Hannezo, M. Reichert#, A.R. Bausch.# Nature Communications (2022) *equal contribution, #corresponding authors
Selective multi-kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Falcomatà C, Bärthel S, Widholz SA, Schneeweis C, Montero JJ, Toska A, Mir J, Kaltenbacher T, Heetmeyer J, Swietlik JJ, Cheng JY, Teodorescu B, Reichert O, Schmitt C, Grabichler K, Coluccio A, Boniolo F, Veltkamp C, Zukowska M, Vargas AA, Paik WH, Jesinghaus M, Steiger K, Maresch R, Öllinger R, Ammon T, Baranov O, Robles MS, Rechenberger J, Kuster B, Meissner F, Reichert M, Flossdorf M, Rad R, Schmidt-Supprian M, Schneider G, Saur D. Nature Cancer (2022)
Identification of treatment-induced vulnerabilities in pancreatic cancer patients using functional model systems. Peschke K, Jakubowsky H, Schäfer A, Maurer C, Lange S, Orben F, Bernad R, Harder FN, Eiber M, Öllinger R, Steiger K, Schlitter M, Weichert W, Mayr U, Phillip V, Schlag C, Schmid RM, Braren RF, Kong B, Demir IE, Friess H, Rad R, Saur D, Schneider G, Reichert M. EMBO Molecular Medicine (2022)
T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Lesch S, Blumenberg V, Stoiber S, Gottschlich A, Ogonek J, Cadilha BL, Dantes Z, Rataj F, Dorman K, Lutz J, Karches CH, Heise C, Kurzay M, Larimer BM, Grassmann S, Rapp M, Nottebrock A, Kruger S, Tokarew N, Metzger P, Hoerth C, Benmebarek MR, Dhoqina D, Grünmeier R, Seifert M, Oener A, Umut Ö, Joaquina S, Vimeux L, Tran T, Hank T, Baba T, Huynh D, Megens RTA, Janssen KP, Jastroch M, Lamp D, Ruehland S, Di Pilato M, Pruessmann JN, Thomas M, Marr C, Ormanns S, Reischer A, Hristov M, Tartour E, Donnadieu E, Rothenfusser S, Duewell P, König LM, Schnurr M, Subklewe M, Liss AS, Halama N, Reichert M, Mempel TR, Endres S, Kobold S. T Nature Biomedical Engineering (2021)
Mesenchymal Plasticity Regulated by Prrx1 Drives Aggressive Pancreatic Cancer Biology. Feldmann K, Maurer C, Peschke K, Teller S, Schuck K, Steiger K, Engleitner T, Öllinger R, Nomura A, Wirges N, Papargyriou A, Jahan Sarker RS, Ranjan RA, Dantes Z, Weichert W, Rustgi AK, Schmid RM, Rad R, Schneider G, Saur D, Reichert M. Gastroenterology (2021)
Implementing cell-free DNA of pancreatic cancer patient-derived organoids for personalized oncology. Dantes Z, Yen HY, Pfarr N, Winter C, Steiger K, Muckenhuber A, Hennig A, Lange S, Engleitner T, Öllinger R, Maresch R, Orben F, Heid I, Kaissis GA, Shi K, Topping GJ, Stögbauer F, Wirth M, Peschke K, Papargyriou A, Rezaee-Oghazi M, Feldmann K, Schäfer APG, Ranjan R, Lubeseder-Martellato C, Stange DE, Welsch T, Martignoni ME, Ceyhan GO, Friess H, Herner A, Liotta L, Treiber M, von Figura G, Abdelhafez M, Klare P, Schlag C, Algül H, Siveke JT, Braren RF, Weirich G, Weichert W, Saur D, Rad R, Schmid R, Schneider G, Reichert M. JCI Insight (2020)
Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, Ilmer M, Oberstein PE, Angele MK, Deng H, Hayakawa Y, Westphalen CB, Werner J, Remotti H, Reichert M, Tailor YH, Nagar K, Friedman RA, Iuga AC, Olive KP, Wang TC. Cancer Discovery (2018)
Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer. Reichert M#, Bakir B, Moreira L, Pitarresi JR, Feldmann K, Simon L, Suzuki K, Maddipati R, Rhim AD, Schlitter AM, Kriegsmann M, Weichert W, Wirth M, Schuck K, Schneider G, Saur D, Reynolds AB, Klein-Szanto AJ, Pehlivanoglu B, Memis B, Adsay NV, Rustgi AK#. Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer. Developmental Cell (2018) #corresponding authors
Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Takano S, Reichert M#, Bakir B, Das KK, Nishida T, Miyazaki M, Heeg S, Collins MA, Marchand B, Hicks PD, Maitra A, Rustgi AK#. Genes & Development (2016) #corresponding authors (highlighted in Nature Reviews Cancer)
Isolation, culture and genetic manipulation of mouse pancreatic ductal cells. Reichert M, Takano S, Heeg S, Bakir B, Botta GP, Rustgi AK Gregory P. Botta, Anil K. Rustgi. Nature Protocols (2013)
The Prrx1 homeodomain transcription factor plays a central role in pancreatic regeneration and carcinogenesis. Reichert M, Takano S, von Burstin J, Kim SB, Lee JS, Ihida-Stansbury K, Hahn C, Heeg S, Schneider G, Rhim AD, Stanger BZ, Rustgi AK. Genes & Development (2013) (recommended by Faculty of 1000)
Full publication list: PubMed, Google Scholar
Bavarian Cancer Research Center (BZKF)
German Cancer Aid (Deutsche Krebshilfe, DKH)
Deutsche Forschungsgemeinschaft (DFG)
Federal Ministry for Education and Resrach (Bundesministerium für Bildung und Forschung, BMBF)
German Cancer Consortium (Deutsches Konsortium für Translationale Krebsfoschung, DKTK)
Klinikum rechts der Isar
Translational Pancreatic Cancer Research Center
Klinik und Poliklinik für Innere Medizin II
Trogerstraße 32
81675 Munich
Technical University of Munich
Center for Functional Protein Assemblies (CPA)
Ernst-Otto-Fischer-Straße 8
85748 Garching near Munich